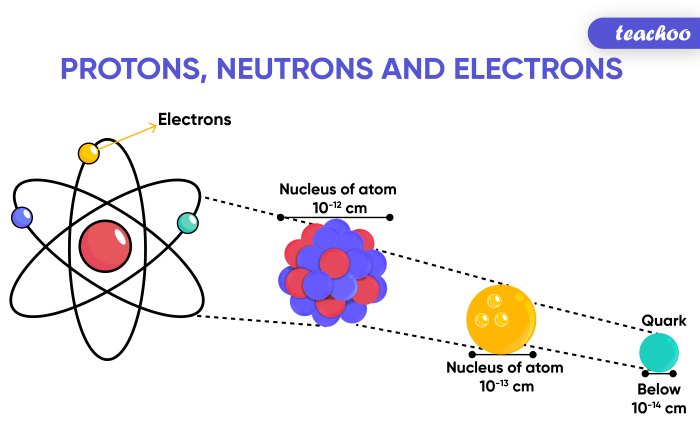
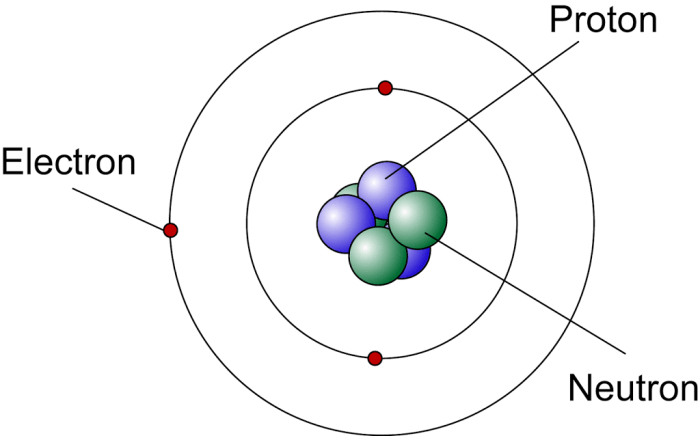
Which statement best describes the nucleus of an aluminum atom – The nucleus of an aluminum atom is the central core of the atom, containing the majority of its mass and a positive charge. It consists of protons and neutrons, which are bound together by the strong nuclear force. The number of protons in the nucleus determines the atomic number of the element, and for aluminum, this number is 13. The nucleus of an aluminum atom is responsible for the atom’s identity and its chemical properties.
Structure of the Aluminum Atom
The aluminum atom is the basic unit of the element aluminum. It consists of a nucleus surrounded by electrons. The nucleus contains protons and neutrons, while the electrons orbit the nucleus in shells.
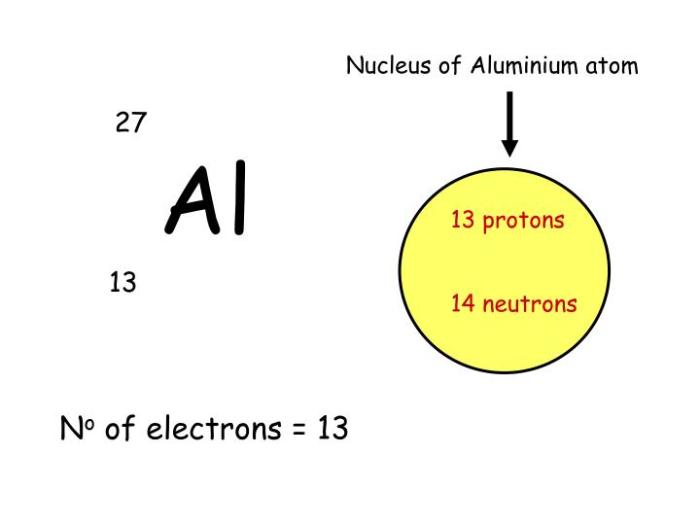
The atomic number of aluminum is 13, which means that the nucleus contains 13 protons. The mass number of aluminum is 27, which means that the nucleus contains 27 nucleons (protons and neutrons).
The electrons in an aluminum atom are arranged in three shells. The first shell contains two electrons, the second shell contains eight electrons, and the third shell contains three electrons.
Nucleus of the Aluminum Atom
The nucleus of an aluminum atom is composed of 13 protons and 14 neutrons. The protons are positively charged, while the neutrons are neutral. The protons and neutrons are held together by the strong nuclear force.
The nucleus of an aluminum atom is very small, with a diameter of about 10^-15 meters. However, the nucleus contains most of the mass of the atom. The electrons, which are much lighter than the protons and neutrons, orbit the nucleus at a relatively large distance.
Properties of the Nucleus
The nucleus of an aluminum atom is very dense, with a density of about 10^17 kg/m^3. This is much denser than the rest of the atom, which has a density of about 2.7 g/cm^3.
The nucleus of an aluminum atom is also very stable. The strong nuclear force is much stronger than the electromagnetic force, which is the force that holds electrons in orbit around the nucleus. This means that the protons and neutrons in the nucleus are very tightly bound together.
Nuclear Reactions Involving Aluminum, Which statement best describes the nucleus of an aluminum atom
The nucleus of an aluminum atom can be involved in a variety of nuclear reactions. One common type of nuclear reaction is radioactive decay. In radioactive decay, the nucleus of an atom emits a particle, such as an alpha particle or a beta particle.
This changes the composition of the nucleus and can lead to the formation of a new element.
Another type of nuclear reaction that can involve the nucleus of an aluminum atom is nuclear fusion. In nuclear fusion, two nuclei combine to form a single nucleus. This process releases a great amount of energy and is the basis for the operation of nuclear reactors and nuclear weapons.
Applications of Aluminum Nucleus
The properties of the aluminum nucleus have a number of practical applications. One important application is in the field of nuclear medicine. Radioactive isotopes of aluminum can be used to diagnose and treat a variety of medical conditions.
Another important application of the aluminum nucleus is in the field of nuclear energy. Aluminum is used as a cladding material for nuclear reactor fuel rods. The aluminum nucleus helps to protect the fuel rods from corrosion and damage.
Essential FAQs: Which Statement Best Describes The Nucleus Of An Aluminum Atom
What is the role of the nucleus in an aluminum atom?
The nucleus is the central core of the atom, containing the majority of its mass and a positive charge. It is responsible for the atom’s identity and its chemical properties.
How many protons are in the nucleus of an aluminum atom?
There are 13 protons in the nucleus of an aluminum atom.
What is the strong nuclear force?
The strong nuclear force is a fundamental force that binds protons and neutrons together in the nucleus of an atom. It is one of the four fundamental forces of nature, along with the electromagnetic force, the weak nuclear force, and gravity.