Acids bases and the ph scale worksheet – Embark on a captivating journey into the realm of acids, bases, and the pH scale through this comprehensive worksheet. Designed to illuminate the intricacies of chemical equilibria, this resource provides a thorough exploration of the fundamental concepts, properties, and applications of acids and bases.
Delve into the Arrhenius theory, unraveling the nature of acids and bases. Discover the characteristics that distinguish these substances, exemplified by their chemical formulas and pH values. Prepare to navigate the pH scale, a crucial tool for measuring the acidity or alkalinity of solutions.
Understand the relationship between pH and hydrogen ion concentration, and classify solutions as acidic, basic, or neutral.
Acids and Bases
Acids and bases are two fundamental classes of chemical compounds that play crucial roles in numerous chemical processes. According to the Arrhenius theory, acids are substances that, when dissolved in water, release hydrogen ions (H +), while bases release hydroxide ions (OH –).
Examples of acids include hydrochloric acid (HCl), sulfuric acid (H 2SO 4), and nitric acid (HNO 3). Bases include sodium hydroxide (NaOH), potassium hydroxide (KOH), and calcium hydroxide (Ca(OH) 2).
Acids and bases have distinct properties. Acids are typically sour to taste, corrosive to skin, and react with metals to produce hydrogen gas. Bases, on the other hand, are bitter to taste, slippery to touch, and react with acids to form salts and water.
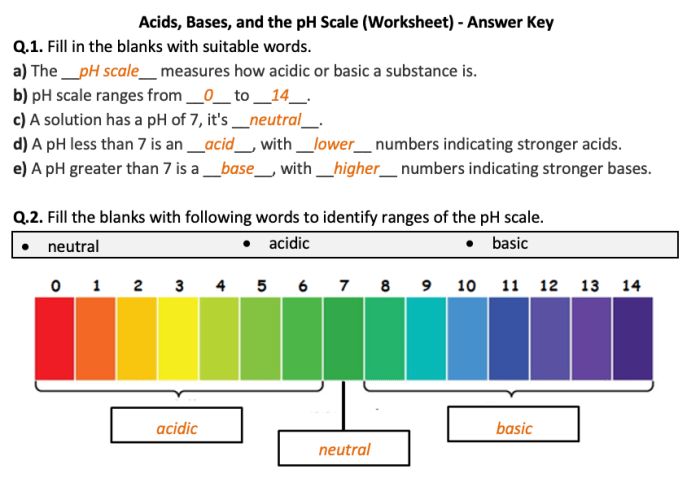
The pH scale is used to measure the acidity or basicity of a solution. It ranges from 0 to 14, with 0 being the most acidic and 14 being the most basic. A pH of 7 is considered neutral.
The pH Scale

The pH scale is a logarithmic scale that measures the hydrogen ion concentration [H +] in a solution. It is calculated as the negative logarithm of the hydrogen ion concentration:
pH =
log[H+]
Solutions with a low pH (less than 7) are acidic, while solutions with a high pH (greater than 7) are basic. Neutral solutions have a pH of 7.
Examples of solutions with different pH values include:
- Battery acid: pH 0
- Lemon juice: pH 2
- Pure water: pH 7
- Household ammonia: pH 11
- Sodium hydroxide solution: pH 14
Acid-Base Reactions
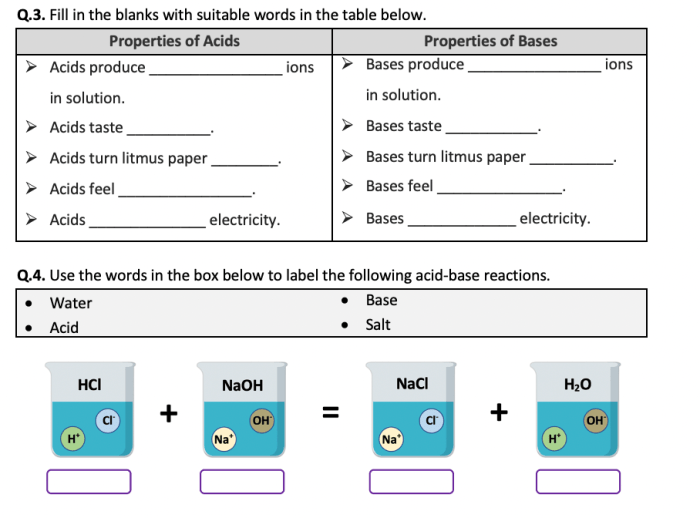
Acid-base reactions are chemical reactions that occur between an acid and a base. The products of an acid-base reaction are typically a salt and water.
The strength of an acid or base determines the extent to which it dissociates in water. Strong acids and bases dissociate completely, while weak acids and bases dissociate only partially.
The pH of a solution can affect the products of an acid-base reaction. In general, a strong acid will react completely with a strong base to form a neutral salt. A weak acid will react partially with a strong base to form a basic salt.
A weak base will react partially with a strong acid to form an acidic salt.
Applications of Acids and Bases
Acids and bases have numerous applications in everyday life and industrial processes. Some examples include:
- Food preservation:Acids are used as preservatives in food to prevent spoilage.
- Water treatment:Acids and bases are used to adjust the pH of water to make it safe for drinking.
- Industrial processes:Acids and bases are used in a wide variety of industrial processes, such as the production of fertilizers, plastics, and textiles.
Acids and bases can also be used to neutralize each other to create buffer solutions. Buffer solutions are solutions that resist changes in pH when small amounts of acid or base are added.
FAQ: Acids Bases And The Ph Scale Worksheet
What is the difference between an acid and a base?
According to the Arrhenius theory, an acid is a substance that dissociates in water to produce hydrogen ions (H+), while a base dissociates to produce hydroxide ions (OH-).
What is the pH scale?
The pH scale is a logarithmic scale used to measure the acidity or alkalinity of a solution. It ranges from 0 to 14, with 0 being the most acidic and 14 being the most basic.
How can I use the pH scale to determine the acidity or basicity of a solution?
To determine the acidity or basicity of a solution using the pH scale, you need to measure the concentration of hydrogen ions (H+) in the solution. A pH value less than 7 indicates an acidic solution, while a pH value greater than 7 indicates a basic solution.